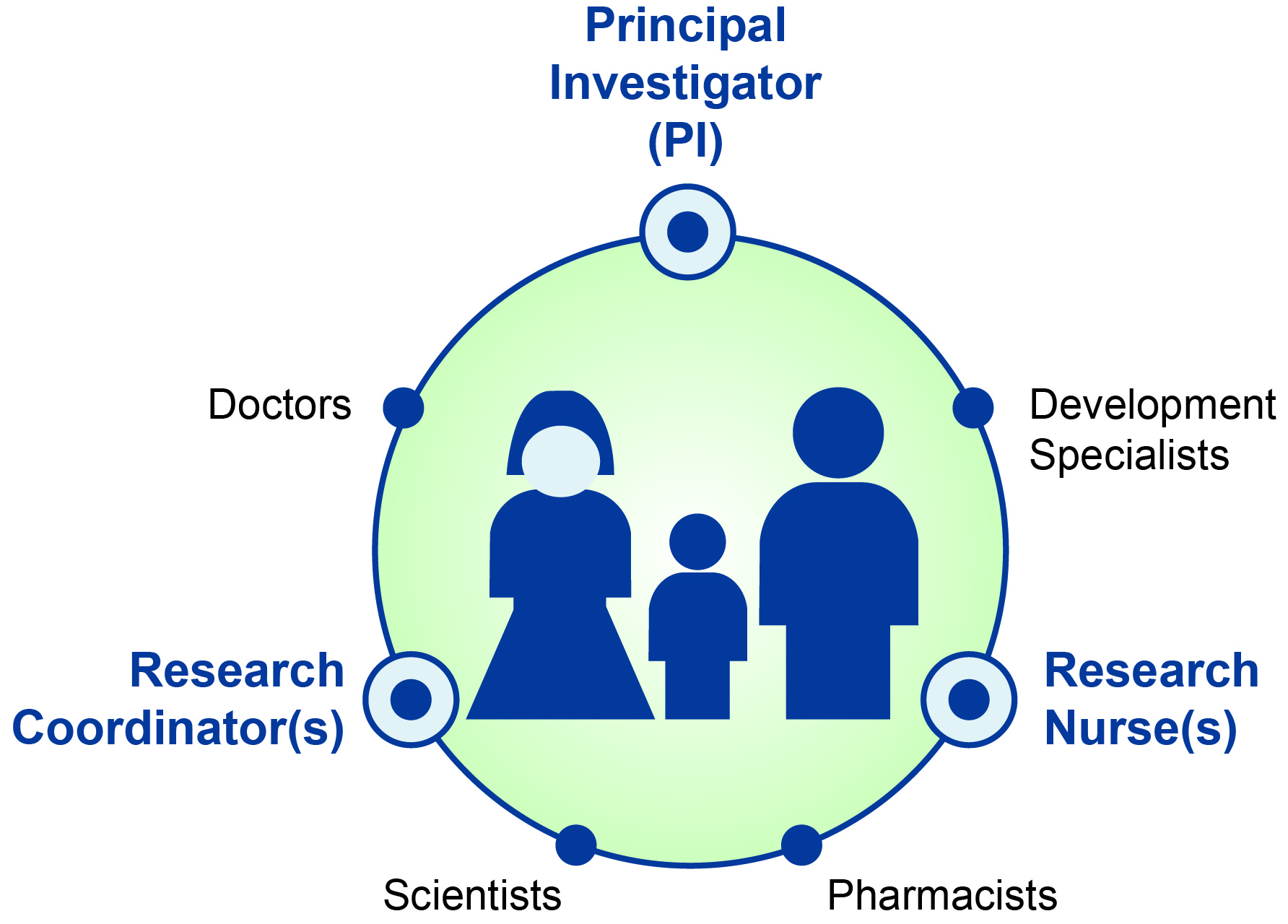
Every clinical study has its own research team.
It’s made up of doctors, nurses, research coordinators and scientists, pharmacists, and developmental specialists, to name a few.
The most important members? Children and their parents.
The medical and research teams make sure that your child is eligible to join a study, give instructions, and monitor your child during the study. Not just anyone who is a nurse or doctor can be on the research team. In addition to their medical training, they must take special classes and are highly trained in how to protect participants and conduct studies in an ethical, legal, and safe way. They are prepared to answer any and all of your questions and they always respect the parent’s role as the one who knows his or her child best.
Parents are at the center of any research team.
Some studies have large teams, and others are small, with one or two individuals managing the study.
The Principal Investigator (PI) is often a medical doctor and is responsible for the entire study. He or she oversees the care of all the participants while they are in the study. The PI can sometimes be your child’s regular doctor.
The Research Nurse(s) are responsible for patient recruitment. They also assess eligibility and, along with the study doctor, provide treatment. Part of their role is to collect data and followup with participants.
The Research Coordinator(s) make sure the study runs smoothly. They understand all parts of the study and make sure the staff has the right equipment, medication, or devices needed to run the study.
Parents should be comfortable and familiar with the study team. They should know who to call for general questions and who to call in an emergency.
What happens if you run out of study drug? Who do you contact about side effects? What if it is 2 a.m.?
There are many people involved in a research team that a parent may not talk to but who play a crucial role. Institutional review boards, study sponsors, oversight committees, and federal regulators often interact with study teams during the study.
Siblings and other family members are also part of the team since they may be affected by the requirements of a study and may have special concerns.